CLINICAL TRIALS + NEW TREATMENTS
Contribute to research and access potential new treatments
Until recently, there was no known way to slow cognitive decline, but promising new treatments are proliferating. BrainHealth can help you learn about appropriate clinical trials and potentially prequalify for new drugs and therapies.
What are Clinical Research Trials?
Before a new drug or therapy is approved to treat a disease, its safety and effectiveness must be carefully tested—first in a laboratory, then on people—in what is called a clinical research trial. Participants in clinical trials can often gain access to potentially effective new treatments as well as participate in front-line disease research and prevention.
Why Should I Participate in a Clinical Trial?
Depending on the trial, there are numerous potential benefits for participants:
-
Gaining access to new treatments before they are publicly available
-
Joining in the global effort to find treatments and cures for disease
-
Taking an active and empowering role in one’s own plan of care and treatment
-
Receiving high quality care and advice from specialists involved in the trial
-
Continuing beneficial treatment and medication even after the trial is over
-
Finding a community of mutual support among other trial participants
Are Clinical Trials Safe?
Every clinical trial follows a strict protocol designed to protect the safety of the study participants. Additionally, the federal government mandates that all clinical trials be overseen by an Institutional Review Board (IRB) made up of physicians, statisticians and members of the medical community. The IRB is appointed in order to ensure that the trial is ethical, that risks are minimal, and that potential benefits offset the risks.
The policy of “informed consent” dictates that each person involved in the study must be fully informed of the clinical trial’s methods, potential benefits and risks before agreeing to participate.

Are There Drawbacks to Participating in Clinical Trials?
Participants in clinical drug trials are often required to discontinue or abstain from other drugs and treatments. With certain drug-responsive diseases or conditions, this can be a serious consideration. However, since there are not yet many highly effective drugs or medical treatments for Alzheimer’s available, this concern is largely eliminated in the case of clinical trials for Alzheimer’s and dementia treatments.
Many clinical drug trials are blind, which means that some participants receive placebos rather than the trial drug, without knowing which they have received. In double-blind studies, researchers also don’t know which participants have received placebos. In all cases, trials are carefully designed to minimize negative impacts on participants receiving the placebo.

What if I want to help Alzheimer’s research but don’t want to take an experimental drug?
There are lots of clinical trials for therapies, treatments and lifestyle changes that don’t involve drugs. Some evaluate the effects of exercise, diet, sleep, and meditation. Others assess education strategies.
Partnership with Global Alzheimer's Platform
The Global Alzheimer's Platform (GAP) is a non-profit organization dedicated to speeding the delivery of innovative therapies to those afflicted with Alzheimer's by reducing the time and cost of Alzheimer’s disease (AD) clinical trials.
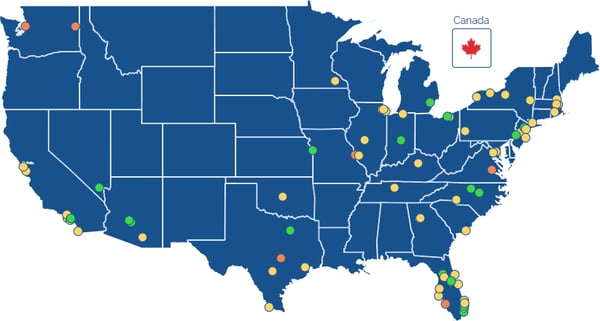
Their network of over 90 clinical trial sites across North America share knowledge and experience in order to effectively and efficiently work toward research progress in neurodegenerative conditions.
BrainHealth is proud to partner with Global Alzheimer's Platform to help place patients in clinical trials that match their biomarker profiles.
Information About Emerging Drug Treatments
Amyloid-targeting antibodies: It is believed that amyloid plaques in the brain build up to contribute in the development of Alzheimer's disease. These treatments use antibodies to target beta-amyloid proteins.
- Eisai: Lecanemab
- Eli Lilly: Donanemab
Tau-targeting drugs: Another type of protein, called tau, has also been linked to Alzheimer's disease. Researchers are currently developing drugs that target tau tangles in the brain.
Anti-inflammatory drugs: Chronic inflammation in the brain is believed to contribute to the development of Alzheimer's disease.
Neurotrophic factors: These are proteins that promote the growth and survival of nerve cells in the brain. Some researchers are exploring the use of neurotrophic factors as a potential treatment for Alzheimer's disease.
It's important to note that many of these emerging drug treatments are still in the early stages of development and may not be available for several years. Additionally, more research is needed to fully understand their effectiveness and potential side effects.
FAQs
If a trial’s results are promising, it will move on to a next phase of study, often involving new trials. If results seem unsafe or unpromising, testing will end. In all cases, results are published, treatments are found worthy of further study or else ruled out, knowledge is gained, and progress is made toward more effective treatments.